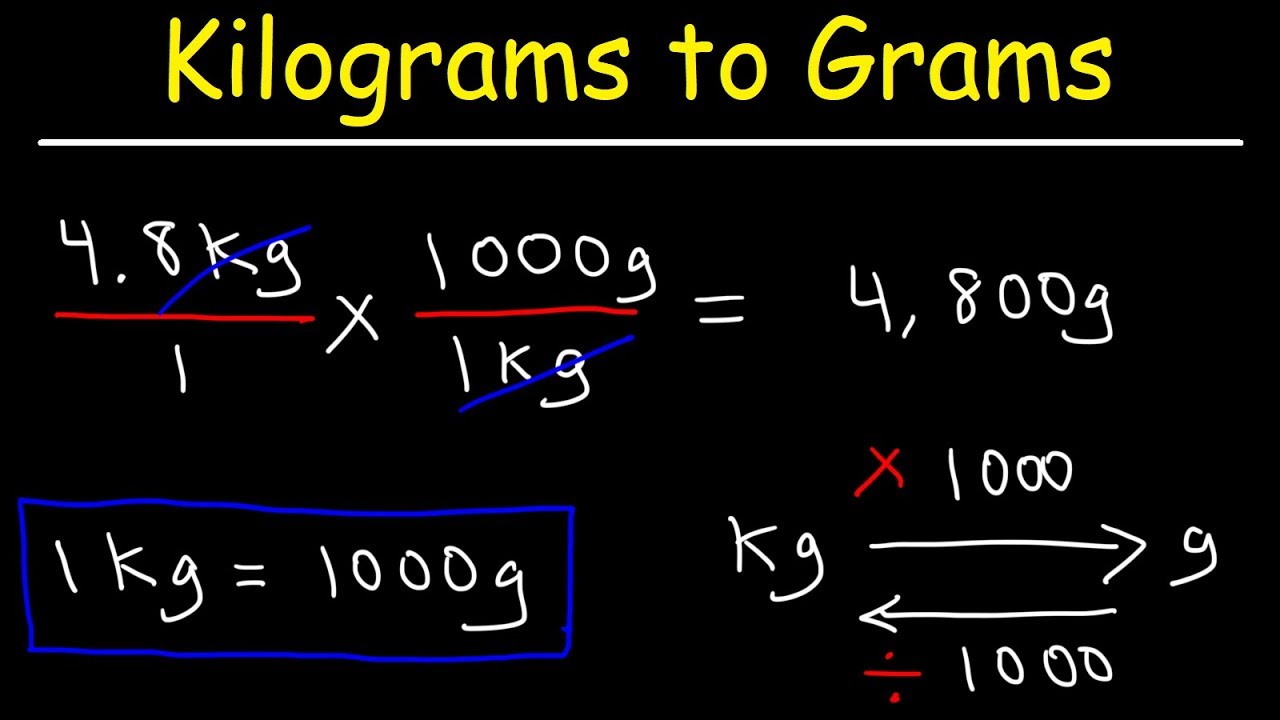The abbreviation "kg" represents the kilogram, a unit of mass in the International System of Units (SI). The term "ng" (or sometimes "ng") typically represents the nanogram, a unit of mass equal to one billionth of a gram. A combination of these units, while not a standard unit, might be encountered in scientific contexts to express a specific mass range.
The use of these units, particularly in scientific, medical, and industrial contexts, highlights the importance of precise measurement in many fields. Accurate quantification, whether in milligrams, kilograms, or nano-levels, allows for detailed analysis, precise dosage control, and reproducible experimental results. Historical context demonstrates a consistent progression towards finer scales of measurement, addressing the growing need for nuanced quantification in specialized applications. This need stems from increasingly sophisticated instruments and advanced research methods. Consequently, the consistent application of appropriate units is paramount to accurately conveying information and ensuring effective communication.
This understanding of mass measurement is fundamental to the topics covered in this article, which will explore [mention specific article topics, e.g., the applications of nanomaterials in medicine, or the analysis of trace elements in environmental samples].
kg ng
Understanding the relationship between mass measurements in kilograms (kg) and nanograms (ng) is crucial for various scientific and industrial applications. These units represent distinct scales of measurement, highlighting the need for precision in quantification.
- Mass measurement
- Precision quantification
- Trace elements
- Nanomaterials
- Dosage control
- Scientific research
- Industrial processes
The key aspects above encompass the importance of accurate mass measurement, crucial for precise quantification in areas like scientific research (e.g., analyzing trace elements in environmental samples) and industrial processes (e.g., controlling dosage in pharmaceuticals). Nanomaterials often operate at the ng scale, demanding meticulous control. Relationships between kg and ng scales are observed in various fields, from understanding the mass of large structures to measuring the tiny components that impact their functionality. For example, the mass of a manufactured component (kg) relies on the precise properties of its nano-scale constituents (ng), highlighting the interconnectedness of these measurement scales.
1. Mass measurement
Accurate mass measurement is fundamental to countless scientific, industrial, and medical applications. The ability to quantify mass precisely, from kilograms to nanograms, underpins critical analyses, enabling progress across diverse disciplines. Understanding the relationship between these vastly different scales of mass measurement is crucial for comprehending the intricacies of phenomena ranging from large-scale structural integrity to the behavior of minute components.
- Precision and Accuracy
Precise and accurate mass measurement is essential for establishing reliable data and drawing meaningful conclusions. In scientific experiments, minute variations in mass can significantly impact outcomes, highlighting the necessity for instruments capable of measuring mass with high precision, particularly when dealing with quantities on the nanogram scale. Calibration and validation of measurement tools are paramount to ensuring consistency and reliability, contributing directly to accurate analysis.
- Scaling and Proportionality
The relationship between mass measurements at the kilogram and nanogram scales emphasizes proportionality and scaling principles in various contexts. A structure's overall mass (kilograms) relies on the collective mass of its constituent elements (potentially including materials measured in nanograms), and this relationship dictates its behavior and properties. This concept is critical in engineering design, material science, and manufacturing.
- Application in Material Science and Engineering
Material science relies heavily on precise mass measurements to understand the composition and properties of materials. Analyzing trace elements in materialssometimes quantified in nanogramsreveals key information about their performance characteristics, while the macroscopic mass (kilograms) allows for an understanding of bulk properties and behavior.
- Clinical and Diagnostic Applications
In medical contexts, precise mass measurements are vital for various diagnoses and treatments. Accurate dosage administration, frequently measured in milligrams or micrograms, relies on precision in mass measurement. Similarly, analyses of biological samples often involve the measurement of components at the nanogram level for disease detection and personalized medicine.
In summary, mass measurement, from kilograms to nanograms, plays a multifaceted role in diverse applications. Precise quantification at all scales is essential for scientific understanding, engineering design, industrial processes, and medical diagnostics. The connection between macroscopic and microscopic mass measurements highlights the interconnected nature of phenomena across various scales and underscores the importance of accurate quantification for a comprehensive understanding of the natural world and technological advancements.
2. Precision Quantification
Precision quantification, the act of determining a quantity with high accuracy and repeatability, is intrinsically linked to the units kg and ng. The ability to measure mass precisely, spanning several orders of magnitude from kilograms to nanograms, underpins numerous scientific, industrial, and medical advancements. Accurate measurements are critical for understanding material properties, controlling processes, and ensuring safety in applications ranging from large-scale manufacturing to microscopic biological studies.
The relationship between kg and ng highlights the necessity for precision across diverse scales. In material science, precise measurements of both bulk materials (kg) and trace elements (ng) are essential to understand the correlations between macroscopic properties and microscopic composition. For example, the strength of a metal alloy (kg scale) depends on the precise arrangement and quantity (ng scale) of its constituent elements. In pharmaceuticals, precise quantification of active ingredients at the nanogram level is critical for effective drug delivery and minimizing side effects. Likewise, in environmental monitoring, precise quantification of pollutants at the nanogram level (e.g., heavy metals) is crucial for assessing environmental impact and developing effective remediation strategies.
Challenges remain. Developing instruments capable of consistently measuring minute quantities with high precision, especially in complex matrices, necessitates ongoing advancements in technology and methodology. Furthermore, the interpretation of precise measurements requires a strong understanding of the underlying principles, context, and potential sources of error. A comprehensive understanding of precision quantification, encompassing both the technical capabilities and contextual interpretations, is essential for harnessing the full potential of kg and ng measurements across diverse fields.
3. Trace elements
Trace elements, present in minute quantities, often in the nanogram (ng) range, exert significant influence on the properties and behavior of materials, systems, and organisms. Their presence in quantities measured in kilograms (kg) is, of course, negligible and irrelevant. Their influence, however, is profoundly impactful. Understanding the relationship between their quantities and their effects requires precision measurement, utilizing instruments capable of handling such minute concentrations.
- Quantification and Analysis
Precise quantification of trace elements is critical for understanding their impact. Analytical techniques capable of detecting and measuring these elements at the nanogram level are essential. Methods like atomic absorption spectroscopy and inductively coupled plasma mass spectrometry are vital for accurate analyses, supporting research and applications in diverse fields.
- Impact on Material Properties
Trace elements can significantly modify the physical and chemical properties of materials. Their presence at the nanogram level can affect the strength, durability, conductivity, and other characteristics of metals, alloys, and compounds. For instance, small additions of certain trace elements can enhance the corrosion resistance of steel or improve the catalytic activity of a material, as specific elements have distinctive influences at the atomic level.
- Biological Significance
Trace elements are indispensable for biological processes. Their presence in organisms, measured frequently in nanograms, is essential for enzyme function, metabolic processes, and cellular structure. Deficiencies or excesses of these elements can have profound effects on health and development. Maintaining optimal levels is crucial for well-being.
- Environmental Implications
Trace elements in the environment, though often present at the nanogram level in specific locations, can accumulate and impact ecosystems. Understanding their concentrations and distribution is essential for assessing environmental health. Concentrations can originate from various sources and influence the health and productivity of local flora and fauna.
In conclusion, the precise measurement of trace elements, frequently using units of nanograms, is crucial for comprehending their effects in materials, biological systems, and the environment. Their influence, though often measured at extremely low levels, underscores the importance of precise quantification to reveal the relationships between structure, function, and behavior at microscopic and macroscopic scales. The influence of trace elements (measured in ng) is essential for determining the behavior of materials, organisms, and environments at much larger scales (measured in kg).
4. Nanomaterials
Nanomaterials, characterized by dimensions typically measured in nanometers, present a unique interplay with mass units like kilograms (kg) and nanograms (ng). The properties and behaviors of these materials are often fundamentally different from their bulk counterparts due to the heightened surface area-to-volume ratio at the nanoscale. This difference in behavior is directly tied to the precision required for quantification, making mass measurement (both kg and ng scales) paramount for understanding and manipulating these materials effectively.
- Composition and Structure
The composition of nanomaterials frequently involves precise control over the proportion of constituent elements. Often, these elements are measured in nanograms, while the overall mass of a product may be in kilograms. The manipulation of these elements in precisely controlled amounts at the nanoscale is crucial for achieving tailored properties. The ability to precisely control and measure the composition of nanomaterials is essential for achieving desired functionalities.
- Surface Area Effects
The heightened surface area-to-volume ratio of nanomaterials dramatically impacts their reactivity and interactions. This phenomenon is directly relevant to mass measurement. The increased surface area enhances reaction rates, making nanomaterials attractive catalysts. Quantifying the mass of these materials, both at the macro and nanoscale (kg and ng), is essential to understand how surface interactions influence behavior.
- Synthesis and Processing
The synthesis and processing of nanomaterials often involve precise control over the growth and assembly of nanostructures. This precise control is deeply connected with the concept of mass measurement in these materials. The amount of precursor materials and their transformation into desired nanomaterials is frequently quantified at the nanogram level. Ultimately, this control over precise quantities enables the creation of unique and valuable products, such as drug delivery systems and advanced electronics, measurable in kilograms.
- Applications in Diverse Fields
Nanomaterials find application in diverse fields, including medicine, electronics, and energy production, frequently spanning from individual nanograms to complete systems measurable in kilograms. For example, in drug delivery, the precise quantities of medication delivered at the nanoscale (ng) are crucial for efficiency and safety. The bulk properties of these combined materials (kg) are also of critical importance.
In summary, understanding the relationship between kg and ng is critical to comprehending the complexities of nanomaterials. Precise measurement at both scales, ranging from nanograms to kilograms, is fundamental for their synthesis, characterization, and application across a wide range of fields. The accurate measurement of nanomaterial properties is essential for understanding and controlling their properties for practical applications.
5. Dosage Control
Dosage control, the precise administration of a therapeutic substance, is inextricably linked to the units kg and ng. Precise dosage is crucial for efficacy and minimizing adverse effects. Quantifying active ingredients at the nanogram (ng) level is vital for targeted therapies. Conversely, the overall therapeutic dose administered (kg level) necessitates careful consideration of the administered substance's characteristics. The precise relationship between these two scales is essential for safe and effective treatment.
Consider pharmaceutical production. Manufacturing processes must meticulously control the concentration of active pharmaceutical ingredients (APIs) to ensure consistent therapeutic efficacy. APIs are often present in nanogram quantities within the dosage form, while the administered dose is measured in milligrams or micrograms. The accuracy of these measurements determines the effectiveness of the medication and minimizes the risk of under- or overdosing. Errors at either the ng or kg level can have significant consequences, from treatment failure to severe toxicity. Real-world examples abound, demonstrating that accurate dosage control is a direct result of the precise quantification, from trace amounts to whole doses, and understanding this quantitative relationship is critical. The scale of measurement must be properly considered, emphasizing accuracy throughout the entire process, from ingredient manufacturing to final dosage form.
Accurate quantification, from the nanogram quantities within a drug compound to the kilogram quantities of the complete dosage, is fundamental for safe and effective medical treatments. The precision required across these orders of magnitude underscores the importance of meticulous standardization, rigorous testing, and quality control measures throughout the manufacturing chain. Maintaining consistency in dosage control, linked to precise mass measurement across these different scales, directly impacts the reliability and safety of therapeutic outcomes, from managing acute conditions to preventing chronic illnesses. Consequently, the accurate relationship between dosage control and the units kg and ng is paramount for the safety and effectiveness of modern medicine.
6. Scientific research
Scientific research frequently necessitates precise measurements, spanning orders of magnitude from kilograms to nanograms. The ability to quantify phenomena across these scales is essential for understanding complex systems, from the macroscopic behavior of materials to the microscopic interactions within them. This exploration emphasizes the critical role of accurate mass measurements in diverse scientific investigations.
- Quantifying Material Properties
Scientific investigations often involve analyzing the composition and properties of materials. Precise mass measurements, ranging from the overall mass of a sample (kilograms) to the quantities of trace elements (nanograms), are instrumental in determining material characteristics. For instance, determining the precise amount of a particular metal in an alloy (ng) reveals its impact on the alloy's mechanical properties (kg). Such analyses are crucial in fields like metallurgy, materials science, and engineering.
- Understanding Biological Systems
Biological research frequently relies on measuring minute quantities of substances, often in the nanogram range. Determining the concentration of specific molecules (ng) within cells, tissues, or biological fluids is vital for comprehending biochemical pathways, cellular processes, and disease mechanisms. Analyzing the mass of a biological sample (kg) can be used for comparison and interpretation of the biological processes involving the nanogram-level constituents. This applies broadly in molecular biology, biochemistry, and medicine.
- Environmental Monitoring and Analysis
Environmental research often requires measuring pollutants or trace elements at the nanogram level. The concentration of contaminants (ng) in water or soil samples, for instance, is critical for evaluating environmental health and assessing the impact of industrial activities or natural processes. Such measurements are often integrated with analysis of larger-scale environmental samples (kg) for a holistic understanding. This applies to toxicology, environmental chemistry, and ecology.
- Development of Novel Technologies
Developing new technologies often demands accurate measurement at different scales. The precise amounts of specific elements (ng) required for a novel material's synthesis or the performance characteristics of a nano-device (ng scale) need precise determination, whereas the mass of the resultant product (kg scale) needs equivalent scrutiny. This is central to fields like nanotechnology, advanced materials science, and biomedical engineering.
In conclusion, scientific research across disciplines relies on the ability to measure and interpret data spanning the vast scale from nanograms to kilograms. This multifaceted approach enables a comprehensive understanding of phenomena, from the fundamental interactions at the molecular level (ng) to the macroscopic behavior of systems (kg). Accurate measurement is paramount for establishing credible findings, enabling progress in scientific knowledge and technological development.
7. Industrial processes
Industrial processes frequently necessitate precise control over materials and their transformations. This precision extends to quantities spanning many orders of magnitude, from the kilogram scale for bulk materials to the nanogram scale for trace elements. The interplay between these scales is crucial for maintaining efficiency, quality, and safety in manufacturing and production. Accurate measurement of both large and small quantities is essential for achieving consistent results and meeting product specifications. For example, the precise blending of ingredients (measured in kilograms) in the food industry depends on the concentration of key components (measured in nanograms). Similarly, the controlled addition of trace elements to metals (nanograms) influences the strength and durability of the final product (kilograms).
Practical examples further illustrate this connection. In chemical manufacturing, the precise mixing of reactants, often measured in kilograms, requires meticulous control over the concentration of catalysts and additives at the nanogram scale. Variations at this level can significantly impact reaction rates, product yields, and overall process efficiency. In pharmaceutical production, precise measurements of active pharmaceutical ingredients (APIs) at the nanogram level ensure the correct dosage in a final product. The overall mass of the finished medicine (kilograms) necessitates that the API concentration is accurately measured and controlled. Likewise, in electronics manufacturing, the precise incorporation of trace impurities during semiconductor fabrication (nanograms) influences the electrical properties of the chips, which are then assembled and measured in kilograms. These examples highlight how precision in both large- and small-scale measurements drives the efficacy and quality of industrial processes.
The ability to precisely measure and control materials across these disparate scales is critical for innovation in industrial processes. Challenges remain in developing instruments capable of consistently measuring minute quantities with sufficient accuracy. However, advancements in nanotechnology and analytical instrumentation are constantly improving our capacity for precision measurement. This understanding is vital for optimizing processes, ensuring product quality, and achieving environmental sustainability in manufacturing, driving efficiency and profitability. Ultimately, the successful management of industrial processes hinges on a profound understanding of the relationships between quantities measured in kilograms and nanograms.
Frequently Asked Questions
This section addresses common inquiries regarding the units kilogram (kg) and nanogram (ng), their applications, and their relationship in various contexts. These units represent distinct scales of measurement, and accurate understanding is critical for precise quantification across diverse fields.
Question 1: What do the units kg and ng represent?
Answer: kg signifies kilogram, a unit of mass in the International System of Units (SI). ng signifies nanogram, a unit of mass equal to one billionth of a gram. These units are used in diverse scientific and technical contexts to express different scales of mass.
Question 2: Why is precise measurement important for kg and ng?
Answer: Precise measurements are paramount for accurate results in scientific experiments, manufacturing processes, and medical applications. Minute variations in mass, whether in kilograms or nanograms, can significantly impact outcomes, requiring high-precision instruments and techniques.
Question 3: Where are kg and ng commonly used?
Answer: kg is a common unit for measuring macroscopic masses, such as the weight of objects or materials, while ng is frequently used for measuring very small masses, such as trace elements in samples or minute components in nanomaterials.
Question 4: How do kg and ng relate in practical applications?
Answer: The relationship between kg and ng is frequently observed in material science, where the macroscopic properties of a material (kg) depend on the precise composition and behavior of its constituent elements or structures (ng). Similar relationships exist in medicine and environmental science.
Question 5: What are some challenges in measuring kg and ng?
Answer: Measuring extremely small quantities like nanograms presents significant challenges. Specialized instruments and meticulous techniques are required to achieve high precision. Conversely, ensuring accuracy in large-scale measurements, while seemingly less demanding, still requires robust methodology and meticulous calibration to prevent errors.
Question 6: Why is the relationship between kg and ng important?
Answer: The relationship highlights the interconnectedness of phenomena across different scales. Precise quantification at both the kilogram and nanogram levels is crucial for understanding complex systems, whether in material science, biomedical research, or environmental monitoring. This interconnectedness is fundamental to progress in various fields of scientific endeavor.
In summary, accurate measurement across the scales of kilograms and nanograms is essential for gaining a comprehensive understanding of the world around us. This precision fosters scientific discovery, advances in technology, and enhances the accuracy of industrial processes and clinical applications.
The following sections will delve deeper into [mention specific topics related to kg and ng, such as nanomaterials or environmental science].
Tips for Utilizing "kg ng" in Context
This section provides practical guidance for effectively incorporating the units kilogram (kg) and nanogram (ng) into analyses and discussions. Precision and clarity are paramount when dealing with these units, as context dictates appropriate application and interpretation.
Tip 1: Define the Context. Clearly establishing the context within which "kg" and "ng" are employed is essential. Are these units used for describing a bulk material or a trace component? This fundamental distinction is crucial for accurately interpreting results and avoiding misinterpretations.
Tip 2: Employ Appropriate Units. Select the most fitting unit (kg or ng) based on the magnitude of the quantity being measured or discussed. Using kg to describe trace quantities or ng to represent large masses leads to a significant misrepresentation of the data, and may contribute to misinterpretations in analysis.
Tip 3: Maintain Consistent Units. When working with various measurements within a study or experiment, ensure consistency in the chosen units (kg or ng) throughout all calculations and analyses. Inconsistent units create confusion and undermine the reliability of conclusions.
Tip 4: Understand Scaling and Proportionality. Comprehending the relationship between quantities measured in kilograms and nanograms is paramount. Differences in scale necessitate a clear understanding of the proportional relationships between data measured at these disparate magnitudes. Data from different scales need careful consideration, interpretation, and integration.
Tip 5: Employ Standardized Notation. Use consistent and standardized notation for units to minimize ambiguity. This ensures clear communication in scientific writing, avoiding potential misunderstandings. Standardized notation improves readability and facilitates the evaluation of the results.
Tip 6: Verify and Validate Measurements. Employ rigorous procedures for validating and verifying measurements, particularly when working with minute quantities like nanograms. Cross-referencing with established data or other techniques is recommended to ensure the accuracy and reliability of results.
Adherence to these guidelines ensures that analyses utilizing "kg ng" are precise, reliable, and effectively communicate meaningful information. Clear definitions, consistent units, and a nuanced understanding of the relationship between large- and small-scale measurements are foundational to robust conclusions.
These guidelines provide a solid foundation for navigating the complexities of scientific analysis that utilizes both the kilogram (kg) and nanogram (ng) units. The following sections will provide in-depth analyses related to [mention specific areas of application, like nanotechnology, material science, or environmental science].
Conclusion
This exploration of the relationship between kilogram (kg) and nanogram (ng) underscores the significance of precise quantification across diverse scales. The interplay between macroscopic and microscopic measurements is crucial in various fields, including materials science, environmental analysis, and biomedical research. Accurate determination of mass, from kilograms to nanograms, is fundamental to comprehending material properties, biological mechanisms, and environmental impacts. The article highlighted the importance of consistent methodology, proper instrument calibration, and a deep understanding of the relationship between quantities at these different scales. The challenges inherent in measuring at such disparate orders of magnitude were also addressed, emphasizing the need for ongoing technological advancements and rigorous validation procedures.
Precise measurement, irrespective of scale, remains a cornerstone of scientific progress. The ability to accurately quantify phenomena from the macroscopic to the microscopic realms allows for a more profound understanding of the world around us, driving innovation and progress in multiple disciplines. Future research should focus on refining analytical techniques, enhancing instrumentation capabilities, and developing new approaches for integrating measurements across these widely divergent scales. This will ultimately lead to more accurate, insightful interpretations of complex phenomena and advance various fields of knowledge. The profound impact of precision measurement on scientific and technological progress cannot be overstated.
You Might Also Like
Unlocking The Meaning Of A White Lightsaber: Symbolism ExplainedNaughty Trivia: Questions & Answers That'll Shock You!
Cute & Unique Duck Names: 50+ Good Names For Your Duckling
Epic & Hilarious Pranks To Pull On Friends!
Grandmother Quotes: Happy Mother's Day Wishes & More!
Article Recommendations